EMIF NEWSLETTER (MARCH 2017)
March 2017
Use the buttons below to jump to each article in this issue.
To Subscribe to the Newsletter, please complete the Enquiry Form.
| Article 1 EMIF |
Article 2 EMIF-MET |
Article 3 EMIF-AD |
Article 4 EMIF-PLAT |
Article 5 IMI |
Since IMI began in 2008, it has funded many successful programs to improve healthcare across the EU, and EMIF has been no exception. The accomplishments made through EMIF, especially with dementia, metabolic disorders, and data harmonization, have produced a proven model for collaboration and data management in medical research. In doing so, “EMIF is allowing research to happen at a speed and scale that was previously not possible,” says Bart Vannieuwenhuyse, Coordinator of the overall EMIF project, and Senior Director of RMEDS Qualitative Sciences at Janssen. To continue that progress, EMIF and IMI need your support.
The Continuing Need for Usable Data |
1A | |||
Simon Lovestone, Professor of Translational Neuroscience at Oxford University, and the overall joint program lead for EMIF, reveals that “one of the things that EMIF is attempting to resolve is this observation that we all make in science, that there is a vast amount of data that is underutilized.”
Technology has enabled us to create and store mountains of data, but finding and utilizing it tends to be either impossible or impractical. That data exists in two forms. First, electronic health records (EHR) from routine patient care, which provide not only a huge sample size otherwise unattainable in traditional research, but also in-depth observational data collected from the experience of individual patients. Second, research data that has already delivered value for a particular project, but could potentially be re-used for a number of other projects. “EMIF is designed to tackle that,” explains Simon. EMIF makes data visible and provides the tools to access and analyze it.
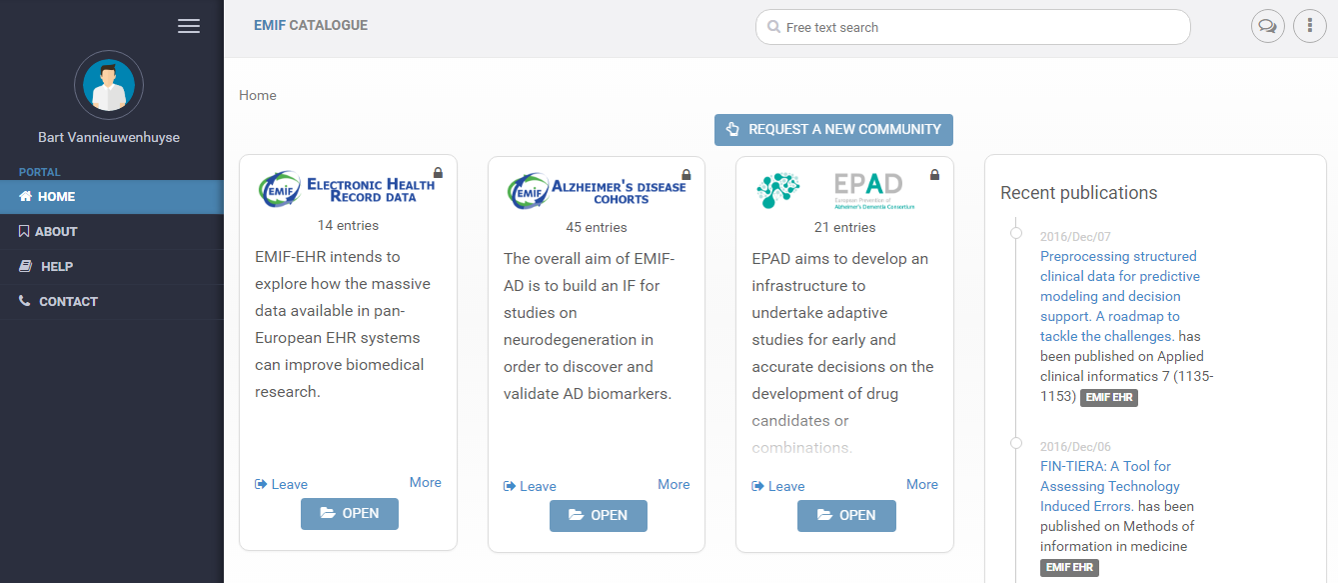
The EMIF Catalogue Home Page includes multiple portals, including the EMIF-AD community, second from the left
A FAIR Goal |
1B | |||
The success of the EMIF initiative has brought medical research to a new level by making data more FAIR (Findable, Accessible, Interpretable, Reusable). To demonstrate this, take the EMIF-AD community in the EMIF Catalogue, which has recently been made available to the public. Through the Catalogue, EMIF has not only made data on Alzheimer’s Disease more visible, but has also created tools to efficiently access and use that data by putting it in a common format. It has been an, “enormously difficult task,” Simon admits, but the Catalogue is already demonstrating value. So far, programs in Europe have already made use of it, including the Dementias Platform UK (DPUK) and the European Prevention of Alzheimer’s Dementia (EPAD), another program funded through IMI.


Take Action |
1C | |||
The impacts of EMIF and IMI have even made their way across the Atlantic Ocean. The 21st Century Cures Act, a United States law enacted by President Obama in December 2016, cites IMI as a successful example to emulate. The important work made possible through EMIF needs to continue. Although EMIF, itself, is finite, it provides a successful model for future collaborative projects in Europe, and the world. Contact Simon or Bart for ideas to extend IMI and EMIF’s impact through the rest of 2017, and beyond.
Regardless of field, EMIF has been a vehicle for researchers to come together in ways that were previously not feasible. That effect has been particularly powerful in the field of metabolics, via EMIF-MET. Two influential members of the metabolics community, Ulf Smith, Professor at the University of Gothenburg, and Project Lead of the Metabolic topic, and Dawn Waterworth, Director of Genetics, Cardiovascular, Metabolic, and Dermatology at GSK, and EFPIA lead of the Metabolic topic, reveal some of the benefits of that enhanced level of collaboration.
Collaboration is King |
2A | |||

Some of the most meaningful discoveries and advancements are not possible if researchers don’t have the infrastructure to go beyond their own field. Dawn explains that in order to get the full value from research, we need to seek out a variety of perspectives. “The lesson [learned from EMIF] is that you have to reach outside your discipline,” she says. EMIF has provided the unique opportunity for researchers across Europe to do exactly that.
In fact, recent a recent bibliographic study has indicated that IMI initiatives receive among the highest citation indexes, and involve collaborations of authors across sectors, industries, nations, and disciplines. Ulf explains that researchers have gained connections that will endure even beyond EMIF. “More than facilitated, it has actually built those connections and relations,” he says.
Taking Discoveries to the Next Level |
2B | |||
In a pre-EMIF research setting, scientists dug deep into small cohorts and identified inspiring possibilities through their projects, “but,” Ulf says, “we don’t get any further than to say, ‘yes, it looks promising in a limited number of individuals.’” When we have the opportunity to work with people who have done that in-depth research within different infrastructures and with different populations, it’s “extremely rewarding,” he says.
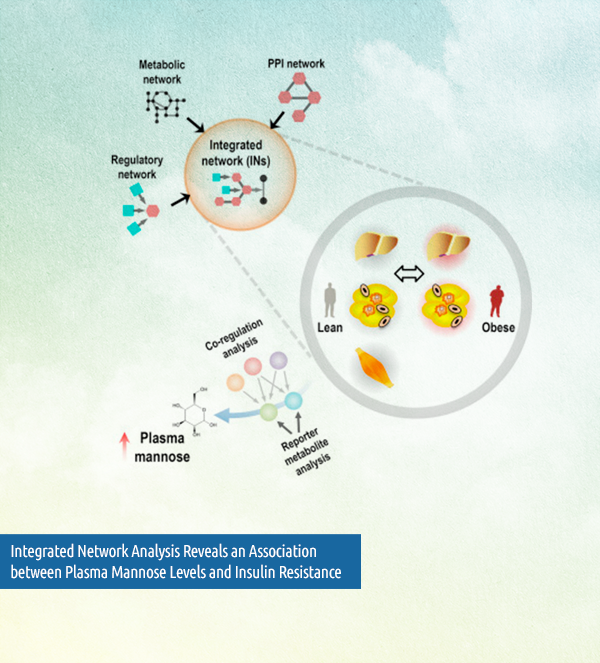
Another rewarding experience are the truly novel results that have only become possible through the recent work of EMIF-MET. Ulf cites a particularly interesting publication which found that mannose is a circulating biomarker of insulin resistance and obesity-associated complications. “We could not have done [this] without the different extremely carefully phenotyped cohorts we collected in the EMIF Metabolic topic,” says Ulf. What’s more, that is only the beginning. In the large follow-up study that will be submitted shortly, “we have identified that mannose predicts future type two diabetes and cardiovascular disease,” he adds.
With over 40,000,000 de-identified patient records from across Europe available, researchers can look at the development of metabolic disease over time, or focus intervention on individuals with high risk. That enables researchers to test, validate, and translate new ideas into valuable concepts that are potentially useful in areas like disease development or therapy. EMIF and IMI make it possible to derive new insights from research.
What’s Next |
2C | |||
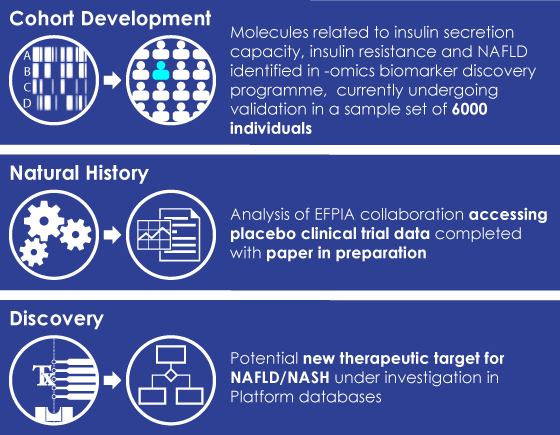
EMIF has produced important work, which, “needs to be maintained,” implores Ulf. The professional relationships that have been cultivated through EMIF will continue even as EMIF begins to wind down. A scientist in genetics, like Dawn, might have a wide network within the field of genetics, but EMIF and IMI “broaden my reach,” she says, adding that, “it allows you to do better, more expansive science at the end of the day.” That is why these collaborations need to continue, whether by supporting new IMI initiatives, or by broadening our professional networks.
Left image: EMIF-Metabolic Project Achievements
Take Action |
2D | |||
Keep up with the contacts you have made within EMIF, and continue to take advantage of networking opportunities that push you outside your field or sector. The connections we make will ensure we continue to press forward as a science community and develop innovations through research. Contact Ulf or Dawn to collaborate or network further within—or beyond—the field of metabolic disease.
Medical researchers in Europe are looking at things in new ways thanks to EMIF’s powerful new infrastructure for collaboration. Associate Professor Pieter Jelle Visser at Maastricht University and VU University Medical Center, and Johannes Streffer, Director of Experimental Medicine at Janssen, are both leaders within the Alzheimer’s disease (AD) topic in EMIF, and represent the collaboration between academia and industry that is one of the core achievements of the EMIF initiative. Their insights about the success of EMIF-AD shed light on the path ahead, as well.
The Rewards of Reuse |
3A | |||
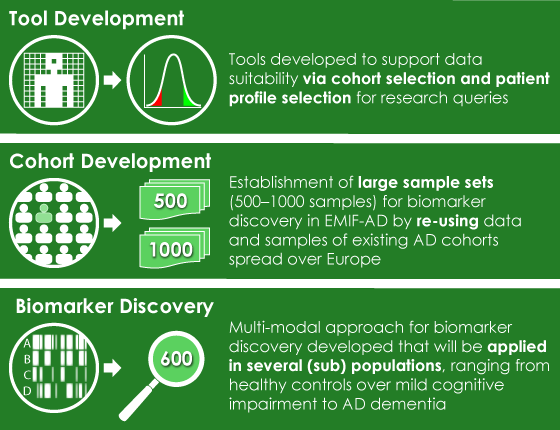
The new infrastructure for collaboration provided by EMIF has already proven to be a valuable tool to those searching for treatments associated with AD, especially as its impact on society continues to grow. “It’s a major disorder with a huge social and economic impact, and there is no treatment yet,” explains Pieter Jelle. That lack of knowledge makes the infrastructure created by EMIF especially important for disorders like AD. It allows us to reuse data that has already been collected, significantly lowering the time and cost required to conduct research. One example is a meta-analysis of 50 cohorts (10,000 subjects) worldwide that was conducted recently, which took only two years. “But,” Pieter Jelle added, “if we had collected 10,000 [subjects] from scratch it would have cost around 25 million Euros, and would have taken something like 10 years.”
Left image: EMIF-AD Project Achievements
Everyone at One Table |
3B | |||
Another major benefit created by EMIF-AD has been “getting industry and academia at one table and aligning on goals,” says Johannes. It has also fostered collaboration between different representatives of the Pharma industry, and even researchers in completely different fields who would never have had incentive to do so before. One benefit of bringing so many parties together is that it allows us to get to know one another’s perspectives. Pieter Jelle explains an example: those in the field of metabolics often focus on how metabolic disorder affects obesity and mortality, while he, as a person working in AD research, is interested in how such disorders could affect the brain.

(left to right) Suzanne Craft, Simon Lovestone, Hilkka Soininen, and Ulf Smith on a panel at the EMIF AD-MET joint meeting in Budapest, March 2016
Take Action |
3C | |||
Pieter Jelle and Johannes are passionate about one topic in particular; that the work being done within EMIF-AD and IMI continues. The infrastructure and technology used to connect the medical research community in Europe has been a wonderful collaborative tool, but it is not just a technological platform. Johannes insists that, beyond the technology involved in EMIF, the collaborative spirit that has been so prevalent needs to endure. That is what will take the success of EMIF to the next level, be it in AD research or beyond.
Keep in touch with the connections you’ve made through EMIF, and reach outside your current research communities to develop new relationships. Contact Pieter Jelle or Johannes with ideas for working across fields and sharing data, in support of AD or other research.
From its inception, the EMIF Platform (EMIF-PLAT) team worked to overcome technical and societal hurdles. Through constant evolution and refinement, they not only created a platform, they created a new way of conceiving what’s possible for shared data in medical science. Already, the results of their work are validating—and inspiring. Nigel Hughes, EFPIA Coordinator of EMIF-PLAT, from Janssen R&D, and Johan Van Der Lei, Academic Coordinator of EMIF-PLAT and Professor at Erasmus University, agree that some of the more human and less measurable outputs have been among the most powerful. The collaborative spirit that brought so many great people together through EMIF will become essential to solving some of the most challenging healthcare and quality of life issues of our time.
Building the Plane, Flying the Plane |
4A | |||
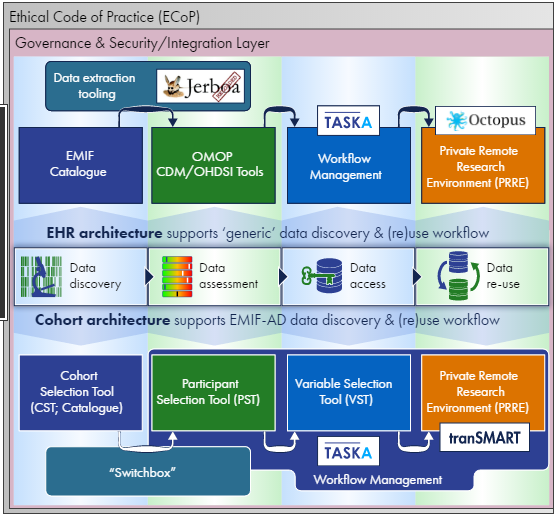
When IMI first decided to merge the AD, MET, and PLAT projects within the EMIF proposal five years ago, the task of data harmonization was met with a degree of skepticism. Data was in the hands of so many different people and organizations, each with disparate ways of storing and managing it. To tackle that, “we are learning as we go, and building as we are learning,” says Nigel. Some of that learning has been about open source technologies and common data models to help systematize and standardize data collection and storage. But some of that learning has been around the value of cross-field and even cross-project collaboration.
In fact, EMIF-AD, EMIF-MET, and EMIF-PLAT, “shouldn’t and can’t exist as separate projects,” says Nigel. Although independent work is sometimes necessary, increasingly, in the second half of the EMIF project, more decisions are being made based on dialogue between projects. For example, the platform was conceived and created at the same time that the Alzheimer’s and metabolic teams were expressing what they needed from the platform. So it was like “trying to fly a plane while you’re still building it,” reveals Nigel. The beauty of that simultaneous process was that the EMIF-PLAT team built what real people and real projects most need.
Left Image: The EMIF-PLAT detailed architectural view reveals two paths: population and cohort. To learn more about the technical aspects of EMIF-PLAT, contact Nigel or Johan, or tune in to the next issue of the EMIF Newsletter.
A Proven Need |
4B | |||
One of the early wins for EMIF-PLAT was the ability to vastly improve the speed with which people can identify data sources. The EMIF Data Catalogue is a systematised, meta-data driven listing of all data sources within EMIF. In January 2017, the EMIF Data Catalogue was released beyond the EMIF community. In less than two months, more than 300 bona fide researchers have already signed up to explore the 14 population data sources and 46 EMIF-AD cohorts. The plan for the coming year, Johan says, is to expand capabilities so that those researchers can evaluate suitability and ultimately conduct studies with data source collaborators.

The EMIF Data Catalogue is now available to bona fide researchers outside of EMIF
The Long and Winding Road |
4C | |||
In many ways, the bumps in the road while building EMIF-PLAT have been more human than technological. “It’s a socio-technical issue!” is something you may have heard if you’ve ever spoken with Johan. Yes, the data is just ones and zeroes, but at the end of the day it’s people’s health data managed by universities, governments, and payers. That means access is also about relationships, laws, and the intended use of that data. “Ultimately it’s about gaining trust,” says Nigel, and to do that, “people need to see something that works.”
Guess What, It Works |
4D | |||
The greatest legacy of EMIF-PLAT will certainly be harmonization. There are already many examples of how harmonizing data vastly improves its usability for research, such as the partnership with the Observational Health Data Sciences and Informatics (OHDSI) collaboration, but EMIF-PLAT also focuses on human harmonization. The most powerful tool that we have available to overcome our most pressing human challenges, medical or otherwise, is one another. EMIF-PLAT is already proving what’s possible. And it’s just the beginning.
Take Action |
4E | |||
We all need to strive for meaningful collaborations. The EMIF-PLAT team looks forward to collaborating with other projects (IMI, IMI2, and others), as well as partners across industries, companies, fields, and sectors, whether public or private. We need to continue pushing for open source technologies and open source ideas to support open science. Instead of withholding lessons learned, Nigel and Johan want to share them. Whether you’re an IT architect, bioinformatics expert, or ethicist, contact Nigel or Johan if you’d like to learn more about the social or technical aspects of EMIF-PLAT, or if you’d like to share ideas about harmonization and how to ensure the legacy of EMIF-PLAT lives on.
Big data analysis holds enormous potential for acquiring new and actionable medical knowledge. The increasing availability of databases containing medical and research data, combined with growing analytic capabilities could lead to better treatments and improved outcomes for patients. As the world’s largest public-private partnership in life sciences, the Innovative Medicines Initiative (IMI) is perfectly placed to harness this potential and lay the groundwork for the reuse of clinical data in Europe.
A History of Success |
5A | |||
Several IMI projects are already working towards this goal. A good example is one of IMI’s older big data projects, EHR4CR, which started in 2011. By connecting electronic health records from 11 different hospitals across Europe, this project created a platform which helps researchers design clinical studies and recruit patients to participate in them. The platform can connect securely to anonymized data within multiple hospital electronic health record systems and clinical data warehouses across Europe, enabling researchers to predict the number of eligible patients for a candidate clinical trial protocol. It also helps them to assess the feasibility of their trials and locate the most relevant hospital sites for recruiting patients.
Another IMI project in this field is eTRIKS, which focuses on improving the translation of data from pre-clinical to clinical studies. It is a service project which uses the open source tool, tranSMART, to provide access to different types of biological and clinical data in a well-structured environment. The project places a special emphasis on data cleaning and standardization to deliver high data quality and improve cross-study compatibility. More than 40 projects have already benefited from its services. Among eTRIKS’s achievements is the creation of the Data Catalogue: a unique repository that contains descriptions of IMI project databases. The catalogue is making it easier for researchers to find information on clinical datasets which may be available for reuse.
And then, of course, there is EMIF, an IMI project which is using the power of big data to solve riddles in two big healthcare challenges of our time: metabolic disorders and Alzheimer’s disease. In order to improve our understanding of these diseases, the EMIF team is connecting a multitude of relevant data sources across Europe. In the process, they’re addressing a number of data issues, including data standards, semantic interoperability, ethics, data privacy, and legal issues. The project is also developing an IT platform which will allow access to multiple data sources and when it’s all done and finished may interlink data from up to 50 million adults across Europe.
On the Horizon |
5B | |||
Looking toward the future, new IMI projects in this field are just starting, as part of IMI’s Big Data for Better Outcomes (BD4BO) program launched in 2016. The program aims to facilitate the use of diverse data sources to deliver outcome measures for treatments that are meaningful for patients, clinicians, regulators, researchers, and others. Disease-specific projects addressing Alzheimer’s disease (ROADMAP), and blood cancers (HARMONY) have already started, and a project on cardiovascular disease will be launched soon. These projects will look at the available clinical data from a multitude of sources, in each of their own disease areas, and then combine, standardize and analyze this data to obtain relevant outcome measures.
Part of the same program is also a newly launched IMI project called DO->IT. Rather than focusing on a specific disease area, this project aims to generalize the results of the other BD4BO projects to all disease areas. One of its main deliverables will be a generic, informed consent form template for participants in clinical trials. The language in this form will be formulated in a way to allow clinical data to be reused for other research purposes. The project will aim to disseminate the form among researchers and companies, so it is used as widely as possible.
Apart from projects that work on these issues directly, IMI wants to instil good data practices into all of its projects. Prior to starting, all projects are required to fill out a data management plan, which encourages teams to think early on about collecting and storing their data according to good standards. The idea, of course, is to ensure that data generated in IMI projects can be potentially combined with data generated elsewhere, and analyzed on a bigger scale.
Take Action |
5C | |||
As we move to an increasingly digital environment in healthcare, but also in society, big data will become a major determinant in how the future of medicine will be shaped. As a public-private partnership, involving a multitude of stakeholders, IMI projects are leading the way in this field, creating a framework in which data across Europe could be shared more easily. The success of IMI projects in this field has the potential to persuade data holders across Europe that all the legal and logistical obstacles of clinical data reuse can be overcome, for the greater benefit of patients.
Contact the IMI infodesk to find out more about—and get involved in—the continuing efforts of IMI.