EMIF NEWSLETTER (MAY 2018)
May 2018
Use the buttons below to jump to each article in this issue.
To Subscribe to the Newsletter, please complete the Enquiry Form.
Bringing Light Where It is Needed |
||||
The space where we need to look for the answers to some of the most pressing questions in medicine today is in the alley, not under the lamppost. The European Medical Information Framework (EMIF) was formed to bring light where it is needed; we wanted to shed light on potential keys to liberating insights and evidence from real world data in Europe.
There is a transformation taking place, with healthcare becoming a truly data-driven sector, from clinical care through to R&D. Compared to many other industries in society, healthcare has been a latecomer to the digital realm. However, over the last five to six years since EMIF was first imagined, healthcare is truly moving into the digital realm, and our project has contributed to that change. In this newsletter—the last of this IMI project—we will be bringing together views from around the EMIF project on what we have achieved, what was a challenge, what lessons we learned, and where we could be going in the near future.
We Can Only Achieve Through Collaboration |
||||
EMIF has been a complex project with its three components: the socio-technical and governance framework (EMIF-PLAT), and the two disease projects, in Alzheimer’s disease (EMIF-AD) and in the metabolic consequences of obesity (EMIF-MET), both working on early biomarker development.
Collectively, we have brought together a remarkably diverse research and development effort within EMIF to address some of the key challenges for modern healthcare. Moreover, we have recognised that we can only achieve through collaboration, working together from when clinical data is generated to when it can potentially be analysed with the latest open-source tools. Our final meeting of the consortium and our public event spoke to the remarkable achievements of EMIF and all those who were involved in it, as well as the challenges and lessons learned. This is covered much further within the newsletter.
We have no doubt that working at scale—with increasingly large cohorts and in collaboration with data custodians internationally and eventually globally—is going to be the standard for real world observational health research. This will especially be the case when cohorts are linked to other complementary datasets, such as the molecular and imaging data used in parts of EMIF. We have learnt much about how to develop and nurture a federated data network, and about the value and indeed challenges of linking such observational data to basic science in the two disease areas. Importantly, we have been learning together. This has transformed not just our knowledge but led to productive and lasting scientific collaborations.
Thank You |
||||
More on the results of EMIF is contained in this newsletter, but we also would want to take the opportunity to thank all those involved in and collaborating with EMIF. The project has set the stage for new projects that are impending, such as the IMI2 European Health Data and Evidence Network (EHDEN), which we hope will start later this year. Meanwhile, sustainable elements of EMIF will be accessible beyond the IMI phase for the research community. EMIF is already contributing to the infrastructure of other projects—providing the EMIF Catalogue and contributing to the harmonisation within Dementias Platform UK for example.
Setting New Precedents |
||||
EMIF has been an outstanding success, as evidenced by one of the highest citation impacts of any IMI project, and its scientific output in the three domains will continue to reverberate for many years. Insights generated into, for instance, the variable progression of Alzheimer’s disease, the liver-related consequences of obesity, and the utilisation of common data models in harmonising diverse European data, have set new precedents.
EMIF has done much to grow the community, platforms, infrastructures, and case for enabling the reuse of data in Europe. We look forward to this legacy being carried forward, not least through other IMI programmes and other initiatives as well as the EMIF continuation plans. All of you who have done so much to make EMIF the success that it is should be as proud as we are of the work we have done. We hope you enjoy reading some of the work of EMIF in this newsletter and many scientific papers to come, shining yet more light on where the keys to medical and societal challenges may be found.
| MAY 2018 |
02 |
|||
Achievements, Lessons Learned, and Opportunities: Reflecting on the Past Five Years of EMIF |
||||

|
KEY POINTS | |||
 |
The European Medical Information Framework (EMIF) project has made major contributions towards promoting the reuse of healthcare data by developing tools for this purpose and by applying these tools in the fields of Alzheimer’s disease and the metabolic complications of obesity. |
|||
 |
EMIF’s mission to improve access and re-use of healthcare data has been accomplished to a large extent, but sustainability will be crucial in order to leverage the full potential of EMIF. |
|||
 |
EMIF has resulted in new collaborations that project leads hope will continue into the future.
|
|||
| CONTRIBUTORS | ||||
 |
NIGEL HUGHESEFPIA Coordinator of EMIF-PLAT, Janssen Pharma R&D  |
|||
 |
ULF SMITHProfessor, University of Gothenburg, Public lead of the Metabolic topic  |
|||
 |
JOHANNES STREFFERVP and Head of Early Clinical Neurology, UCB, EFPIA lead of the AD topic  |
|||
 |
PIETER JELLE VISSERAssociate Professor, Maastricht University, VU University Medical Center, Public lead of the AD topic  |
|||
 |
DAWN WATERWORTHDirector of Genetics, Cardiovascular, Metabolic and Dermatology, GSK, EFPIA lead of the Metabolic topic  |
|||
Achievements Made Possible by EMIF |
||||
Nigel Hughes, the European Federation of Pharmaceutical Industries and Associations (EFPIA) Coordinator of EMIF-Platform, says that EMIF created “a community of disparate and diverse stakeholders with common goals around real-world, observational research.” These stakeholders then fostered the scientific and technical achievements of the project. One of the major achievements of the project is a common information framework, the EMIF Catalogue, to link up and facilitate access to diverse medical and research data sources. Over the past five years, EMIF has created a working platform and mapped multiple databases to the OMOP common data model.
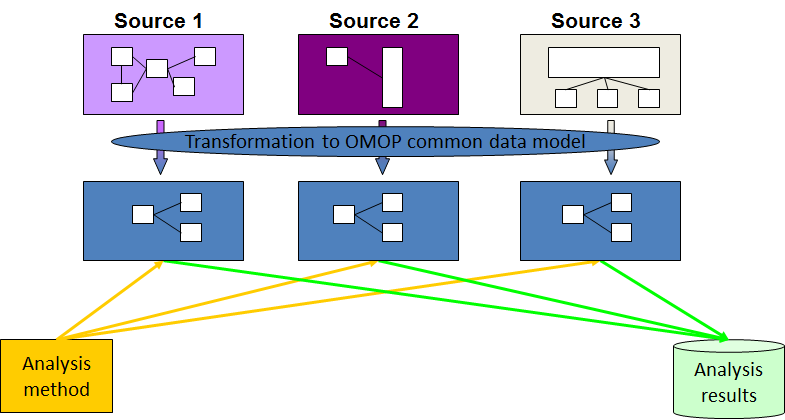
Diagram representing the process of data standardization using the OMOP Common Data Model.
According to Johannes Streffer, the EFPIA lead of EMIF-AD, EMIF fosters and strengthens relationships between and among partners and data providers, which connects research centers more effectively. EMIF-AD has also developed tools such as the EMIF-AD Catalogue (with the cohort and patient selection tools), tranSMART, and Switchbox. Pieter Jelle Visser, public lead of EMIF-AD, notes that these tools are only possible through EMIF because of the combination of different types of expertise. These tools and pooled datasets have enabled EMIF-AD to perform large meta-analyses for a fraction of the cost and in a fraction of time that it would have taken otherwise.
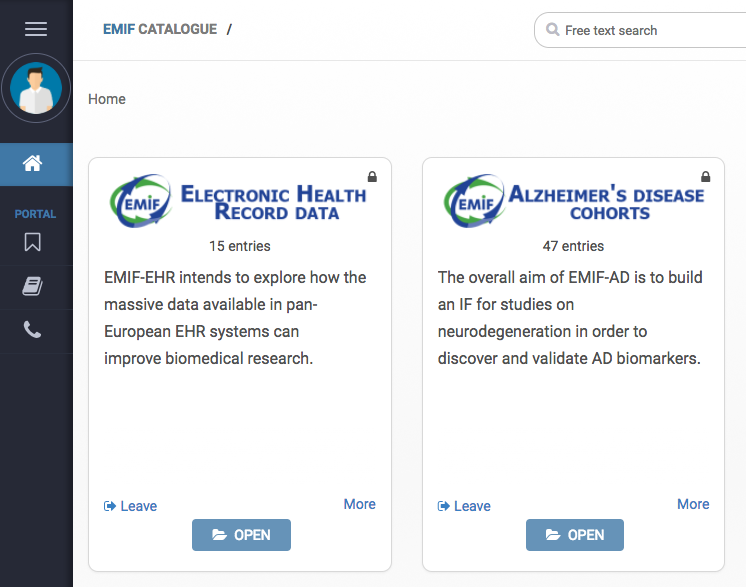
The EMIF-AD Catalogue.
The EFPIA lead of EMIF-Metabolic, Dawn Waterworth, says that “without EMIF, we would not have been able to access electronic medical record (EMR) databases in Europe to run parallel analyses in four databases. We also were able to bring together a multi-disciplinary team that had the appropriate skills and knowledge to take advantage of this data, which would have been very difficult otherwise.” According to Ulf Smith, public lead of EMIF-Metabolic, having scientists with complementary expertise work with a carefully phenotyped cohort has been crucial in validating the importance of novel biomarkers.
Mission Accomplished |
||||
EMIF has achieved its mission of developing common technical and governance solutions and facilitating access and use of healthcare data. “EMIF is helping to liberate insights within Alzheimer’s disease, metabolic diseases and other co-morbidities, from within the project through a series of use cases, and externally via the Catalogue,” says Nigel. The EMIF-AD Catalogue has over 400 registered users, clearly indicating that it meets a strong need.
There remain challenges, especially around governance and provenance of local data and external engagement with stakeholders. All of the topic leads agree that sustainability is essential to continuing EMIF’s mission and to leverage the full potential of EMIF.
Lessons Learned |
||||
The topic leads agree that the time spent building communities within the three areas of EMIF (Platform, AD, and Metabolic) was very valuable. In EMIF-AD, the topic leads are happy with how the teams achieved deliverables clearly and transparently, created cohorts and infrastructures, and re-used existing data. The EMIF-Metabolic topic leads say that their projects have led to some great collaborations, and they are pleased with the access to complementary expertise and large cohorts with disease outcome data.
For future similar projects, the topic leads have some aspirations. One of the keys to building a federated data network for real world, observational research in Europe is gaining an understanding of data sources’ local governance and provenance requirements. Future projects should also focus more on stakeholders, communicating the opportunity and possible rewards to partner organizations and data providers. This would allow better utilization and access of both data and infrastructure. For a long-term project like EMIF, topic leads would advise EFPIA partners to hire people specifically to work on the project, to make sure that they have time available for it.
Beyond EMIF |
||||
All the topic leads agree that the EMIF project resulted in new high-level collaborations that they hope will continue beyond the five-year span of the project. In EMIF-Platform, there was collaboration with the OHDSI community around the OMOP common data model. Within EMIF-AD, there was linkage between, for example, European Prevention of Alzheimer’s Dementia Consortium (IMI EPAD), Dementias Platform UK (DPUK), et al, as well as the wider Alzheimer’s research community. In EMIF-Metabolic, there were collaborations with a number of EFPIA and academic participants that will continue into the future.

Johannes says, “Collaborations within existing partnerships have been strengthened and will be strong drivers of further research initiatives. New partnerships have been built and will hopefully further develop.” Nigel suggests that such developments are already taking place: “I believe that EMIF cemented relationships between academia, industry/EFPIA partners, and data sources in a pre-competitive state. I would suggest that this paved the way for the forthcoming European Health Data and Evidence Network (EHDEN) IMI2 project.” It will be exciting to see the legacy of EMIF in action in the form of projects such as EHDEN.
| MAY 2018 |
03 |
|||
Bridging Europe: The First Annual OHDSI Symposium |
||||

|
KEY POINTS | |||
 |
Over 200 participants attended the first annual European Observational Health Data Sciences and Informatics (OHDSI) symposium, hosted in Rotterdam, The Netherlands, by the EU OHDSI chapter. |
|||
 |
During the first day of the symposium, presenters spoke about the adoption of the Observational Medical Outcomes Partnership (OMOP) common data model (CDM), the need for an EU OHDSI initiative, the importance of OHDSI tools, and more. |
|||
 |
During the second day of the symposium, two workshops gave participants hands-on experience with the OMOP CDM and OHDSI tools.
|
|||
| CONTRIBUTORS | ||||
 |
JELLE PRAET,PhDLife Science consultant at CMAST bvba, subcontractor at Janssen Pharmaceutica  |
|||
 |
PETER RIJNBEEK, PhDAssistant Professor at Erasmus University Medical Center  |
|||
Importance of the OMOP CDM |
||||
George Hripcsak (Vivian Beaumont Allen Professor and Chair, Biomedical Informatics, Columbia University Medical Center; Director, Medical Information Services, NewYork-Presbyterian Hospital) was the first speaker of the day. He presented about the worldwide journey of OHDSI and the adoption of the Observational Medical Outcomes Partnership (OMOP) common data model (CDM). Currently, over 400 million patients and 1.26 billion patient records have been mapped to the OMOP CDM. Among its users are large institutes like the Food and Drug Administration (FDA), the Electronic Medical Records and Genomics Network (eMERGE), the National Institutes of Health (NIH), and the Innovative Medicines Initiative (IMI).
Peter Rijnbeek (Assistant Professor, Department of Medical Informatics, Erasmus MC Rotterdam) elaborated on the need for an EU OHDSI initiative. There are many different database structures and coding systems in Europe, and healthcare systems differ considerably across EU countries. Harmonization to the Standardized Vocabulary of the OMOP CDM is critical to the generation of real world evidence in a fast and reproducible way. The current “one study – one script” approach is not scalable and not transparent; moreover, it is expensive, slow, and prohibitive to non-expert use. The EU OHDSI initiative will stimulate the adoption of the OMOP CDM in Europe, providing a platform for European collaboration to further improve the CDM and vocabularies for the European setting.

Patrick Ryan (Sr. Director and Head, Epidemiology Analytics, Janssen R&D; Adjunct Assistant Professor of Biomedical Informatics, Columbia University) gave the last talk of the first session.
The last talk of the first session was given by Patrick Ryan (Sr. Director and Head, Epidemiology Analytics, Janssen R&D; Adjunct Assistant Professor of Biomedical Informatics, Columbia University). “We want our science to be reproducible by others, which is even harder than being able to repeat your own results,” said Patrick. If we want our science to become reproducible, we need to ensure that every step along the research process is identical. This is exactly why the OMOP CDM and OHDSI tools are so important. They are the product of a community working together to define community standards and tools, share evidence, and define best practices.
Adoption of the OMOP CDM |
||||
After the coffee break, different cases of the adoption of the OMOP CDM were shown. Nigel Hughes (Scientific director, JCI Patient Data for Research, Janssen, Belgium) presented the upcoming IMI European Health Data and Evidence Network (EHDEN) project, which has set the goal of mapping 100 million health records across the EU to the OMOP CDM in the next five years. While this goal is ambitious, Nigel Hughes expressed confidence that they could reach it. EHDEN is not a blank canvas, but builds on what has already been developed in other projects (like, for example, EMIF).

Martin Sedlmayr (Professor, Institute for Medical Informatics and Biometry, TU Dresden, Germany) presented about the Medical Informatics for Research and Care in University Medicine (MIRACUM) consortium.
Martin Sedlmayr (Professor, Institute for Medical Informatics and Biometry, TU Dresden, Germany) introduced the Medical Informatics for Research And Care in University Medicine (MIRACUM) consortium. Recently launched and supported by the German government, the MIRACUM consortium currently represents a quarter of all German university hospitals. It aims to stimulate the reuse of data by adopting the OMOP CDM and the OHDSI tools.
Ben Hughes (Senior Vice President Real-World & Analytics Solutions, IQVIA) discussed why IQVIA, formerly Quintiles IMS, is continuously investing in the OMOP CDM. IQVIA helps many large-scale institutions (such as FDA and the European Medicines Agency) to answer both simple and complex questions about drug safety, adverse-event reporting, and more. Following a trial where 12 databases were harmonized to the OMOP CDM, IQVIA is convinced of the OMOP’s huge potential to help with better research, but IQVIA also remains realistic about the associated costs and time requirements.
Rae Woong Park (Professor, Ajou University School of Medicine, South Korea) presented the journey of how South Korea successfully adopted the OMOP CDM. There are currently many participating hospitals in South Korea with a total of 55 million patients. Despite the efforts of Professor Park, who had been promoting the Korean OHDSI initiative, it took more than 18 months before the first hospitals expressed interest in joining the Korean OHDSI initiative. This change came about in May 2016, due to the Korean National Health Insurance system which started using the OMOP CDM. From this story, we can learn that we need to get EU payers and regulators on board in supporting the EU OHDSI initiative if we want to be successful.
Epidemiological Presentations |
||||
Daniel Prieto (Associate Professor and NIHR Clinician Scientist, Oxford University, UK) introduced the work of his group at Oxford, showing the audience how epidemiological work using patient records can help to study the risks and benefits of medical devices.
Erica Voss (Associate Director, Epidemiology Analytics, Janssen R&D, US) gave an overview of the type of research done at Janssen epidemiology analytics. She explained how real-world data can be used to conduct clinical trial feasibility studies, where you design your trial and then use real world data to validate if your trial is feasible or needs tweaking. She mentioned that “adoption of the OMOP CDM has required a substantial investment of time and money, but it has a very strong return. The more you invest, the more you get out of it.”
Sir Simon Lovestone (Professor of Translational Neuroscience, Oxford University, UK) spoke about the UK Clinical Records Interactive Search (UK-CRIS) system, which holds data from over 2.5 million mental health patients. Most of the data from mental health patients is written down in long text, which poses a tremendous challenge when harmonizing data. Currently, UK-CRIS is implementing a consent for re-contact model where 74% of patients agreed to be contacted again, which will tremendously speed up clinical trials.
OMOP CDM Tools in Action |
||||
After lunch, there was time for several demonstrations of tools related to the OMOP CDM and 40 research posters. The demonstrations included a patient-level prediction package in R, the ARACHNE research network platform, new ATLAS features, the AEGIS tool, and the EMIF Catalogue. The latter was presented by José Luís Oliveira (Associate Professor, University of Aveiro; Vice Director of Institute of Electronics and Informatics Engineering of Aveiro) who showed the audience the complete EMIF Catalogue pipeline from discovery, to assessment, all the way to the re-use of health data.

Prof. Dr. José Luís Oliveira gave a real-time demonstrations of the EMIF Catalogue, an online platform which integrates and enables the discovery and reuse of heterogeneous biomedical databases.
Regulatory Considerations |
||||
During a panel discussion, Jim Slattery (Senior Statistician, European Medicines Agency) mentioned that regulators use observational data more and more to guide decision making. Christian Reich (Vice President of Real World insights, IQVIA) stated that “the lack of reproducible research on which to base decisions is a problem no company, institution, or government can solve on their own, no matter how rich they are.” When asked how the OHDSI community can make sure it is compliant with the new EU General Data Protection Regulation (GDPR), Michel van Speybroeck (Director Data Sciences, Janssen, Belgium) replied that the exact interpretation of the GDPR is debatable, and that these new rules and regulations might be interpreted differently at different levels. However, as the OHDSI approach is to use a federated data network (versus centralized), it will be much easier to be compliant with the new GDPR.

Peter Rijnbeek (Assistant Professor, Department of Medical Informatics, Erasmus MC Rotterdam) closed the first day of the symposium with a hilarious show about the closures of the past OHDSI symposia in the US, including a singing section greatly appreciated by participants.
Hands-on Experience with OHDSI Tools |
||||
The second day of the conference hosted two workshops, allowing participants to get some hands-on experience with the OMOP CDM and OHDSI tools. By the end of the day, participants had a better understanding of the details of the OMOP CDM structure and the Standardized Vocabularies used to harmonize data. The tool ecosystem of the OHDSI community was also shown, with a strong focus on the ATLAS tool, as this allows browsing vocabularies, defining cohorts, estimating risk effects, making patient-level predictions, and more.
More Information |
||||
For more information about the OMOP CDM and the OHDSI community, and to stay up to date about the next edition of this symposium, please visit OHDSI Europe.
| MAY 2018 |
04 |
|||
Achievements and Challenges of a Five Year IMI Project: The Final EMIF General Assembly Meeting and Public Symposium |
||||

|
KEY POINTS | |||
 |
The European Medical Information Framework (EMIF) project held its final general assembly meeting and public symposium in Brussels, Belgium April 16 –18, 2018. |
|||
 |
During the meeting, participants reflected on research in the areas of Alzheimer’s disease and metabolic complications of obesity, as well as sharing posters and demonstrating tools created by EMIF. |
|||
 |
Discussions about legacy and sustainability led to insights that may be helpful in future IMI projects.
|
|||
| CONTRIBUTORS | ||||
 |
JELLE PRAET,PhDLife Science consultant at CMAST bvba, subcontractor at Janssen Pharmaceutica  |
|||
The Journey Thus Far |
||||
The general assembly meeting commenced with Bart Vannieuwenhuyse (Janssen, BE) and Prof Sir Simon Lovestone (University of Oxford, UK) reflecting on the successful journey thus far. EMIF was first conceived in 2011. Since then, EMIF has lived up to its ambition of providing and enabling access to an unprecedented volume of healthcare data, as well as accelerating research in the field of Alzheimer’s disease (AD) and the metabolic complications of obesity.
Nigel Hughes (Janssen, BE) and Johan van der Lei (Erasmus MC, NL) reflected on the social and technical evolution of EMIF during its project lifetime. “One of the major challenges of EMIF has been the fact that we had to fly the plane while building it,” said Nigel. Part of the success of EMIF stems from having three topics together in one overarching project, but this also introduced some challenges, such as defining a common goal across the three topics and between academic and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners. A major legacy of EMIF will be the development of the EMIF platform and its associated tools, as well as the introduction of the OMOP common data model (CDM) in Europe.
AD and Metabolic Successes |
||||
Johannes Streffer (UCB, BE) and Pieter-Jelle Visser (Maastricht University, NL) explained how EMIF-AD has focused heavily on the re-use and enrichment of AD cohort data. This in turn delivered new insights into the pathophysiology of AD and helped to identify potential new AD biomarkers. Exemplars of this focused effort are the AD cohort catalogue, the enriched EMIF Biomarker discovery cohort, and the establishment of two new cohorts on the extreme phenotypes of AD. When asked what they would do differently if they were to redo the project, Johannes and Pieter-Jelle replied that the political and legal dimension of working with real world data should not be overlooked, but instead be tackled as soon as possible in future projects.
Ulf Smith (University of Gothenburg, Sweden) and Dawn Waterworth (GSK, US) stated that the project structure with three different topics has also been critical to the success of EMIF-Metabolic. There was a shared interest with EMIF-AD to study the link between dementia and insulin resistance, and this kind of research was possible due to the collaboration with EMIF-Platform. Working together has enabled us to identify mannose as a new marker for insulin resistance, and to answer critical questions about the risk factors that link to the progression of non-alcoholic fatty liver disease (NAFLD), cardiovascular disease (CVD), and heart failure.

Dawn Waterworth (GSK, US) presented her journey as a geneticist working in the field of real world data.
Poster Prizes and Tool Demonstrations |
||||
The first day ended with a poster session. In addition to the 28 posters, there were also three real-time demonstrations of the tools developed in EMIF. Myriam Alexander (formerly GSK, UK) took home the jury’s poster prize while the public’s poster prize was awarded to Stephen Carter (University of Manchester, UK).
Cohort Research |
||||
The second day of the general assembly meeting started with a session about all the work undertaken that relates to using cohort-related data.
- Isabelle Bos (Maastricht University, NL) and Sarah Westwood (University of Oxford, UK) presented the outcomes of the cerebrospinal fluid (CSF) analyses and the proteomic analyses which were done in the context of the multimodal biomarker discovery study.
- Anouk den Braber (Vrije Universiteit Amsterdam, NL) presented the results of the EMIF 90+ study and the PreclinAD study, two cohorts focussing on the extreme phenotypes of AD.
- Claudia Langenberg (University of Cambridge, UK) and Luca Lotta (University of Cambridge, UK) presented their work on using cohort data to advance type 2 diabetes biomarkers from mere association to a causal relationship.
- Naveed Sattar (University of Glasgow, UK) introduced the audience to the GlasVEGAs study, where the metabolic reaction of Europeans and South Asian men is compared after quickly gaining enforced weight.
- Michel van Speybroek (Janssen, BE) and Rudi Verbeeck (Janssen, BE) explained how they performed the harmonisation of AD cohort datasets to a minimal dataset, hosted on the tranSMART platform. An update was also given on the AD cohort explorer and the associated AD Switchbox, which are being developed to make AD cohort data available to the wider research community.
Upcoming White Paper |
||||
After the break, there was an interactive workshop, moderated by Caroline Sage (CMAST bvba, BE). The workshop asked participants to reflect on how they experienced EMIF and the lessons they had learned. The outcome of the workshop will be combined with the results of an earlier survey conducted within EMIF and then published as a white paper.

During the interactive workshop, people were split into eight different groups, then asked to reflect on how they experienced EMIF and how the field will continue to evolve in the years to come.
EHR Research |
||||
After lunch, there was a session outlining the use of electronic health record (EHR) data.
- Naveed Sattar (University of Glasgow, UK) presented the results of a study in which they investigated the risk of developing cardiovascular disease in NAFLD patients.
- William Alazawi (Queen Mary University of London, UK) showed us the gap in diagnosis and disease assessment of NAFLD between literature and EHR databases.
- Prof Sir Simon Lovestone (University of Oxford, UK) explained how his group has been studying the link between dementia and inflammation, specifically the effect of the anti-inflammatory drug methotrexate.
- Rob Stewart (King’s College London, UK) showed us his work about using EHR data to study dementia prevalence and vascular risk factors for dementia.
- José Luís Oliveira (University of Aveiro, Portugal) guided us through the many different aspects of the EMIF Catalogue (user profiles, database plugins such as Jerboa and ACHILLES, and the community plugins such as TASKA and ATLAS). Glen James (GSK, UK) told us about his experience of using the EMIF Catalogue to answer actual research questions.
- Peter Rijnbeek (Erasmus MC, NL) presented the Jerboa Reloaded tool which was developed within EMIF. Thereafter, he switched gears and explained the work done on the OMOP CDM. In addition, he reported back on a very successful Observational Health Data Sciences and Informatics (OHDSI) meeting in Rotterdam, in March, as a kick-off to the EU OHDSI chapter.

Peter Rijnbeek (Erasmus MC, NL) holding the microphone during a Q&A session on how other projects besides EMIF are helping to share the European health data ecosystem.
The Legacy Scenario |
||||
During the last session of the day, Nigel Hughes presented the legacy scenario of EMIF, followed by a panel discussion on the sustainability of EMIF. Panelists included Bart Vannieuwenhuyse, Prof Sir Simon Lovestone, Pieter-Jelle Visser, Johannes Streffer, Johan van der Lei, Dawn Waterworth, and Katrina Loomis (Pfizer, US). As with many other Innovative Medicines Initiative (IMI) projects, moving towards a sustainable entity has proven difficult. The general sentiment was that the difficulty was largely due to the many different stakeholders involved rather than a technological problem. Lastly, the upcoming IMI project European Health Data & Evidence Network (EHDEN) was an often-recurring topic throughout the discussion; EHDEN can be considered the heir to EMIF, and thus a big part of the EMIF legacy.
Well-Attended Public Symposium |
||||

On Wednesday, April 18, over 110 participants attended the EMIF public symposium “Liberating Evidence from European Health Data.” The day started with an introduction to EMIF by Bart Vannieuwenhuyse and Prof Sir Simon Lovestone. Thereafter, the respective topic leads gave a high-level overview of the tools and technologies which were developed in EMIF (Nigel Hughes), and the new disease insights that were gained in the field of AD (Johannes Streffer) and about the metabolic complications of obesity (Dawn Waterworth).
 Left: Bart Vannieuwenhuyse (Janssen, Belgium) gave the audience a warm welcome on April 18 and started the public symposium by giving a high-level introduction to the EMIF project.
Left: Bart Vannieuwenhuyse (Janssen, Belgium) gave the audience a warm welcome on April 18 and started the public symposium by giving a high-level introduction to the EMIF project.
“A Perfect Storm” |
||||
The next session focused on other projects and how these are currently impacting the European health data ecosystem. Peter Rijnbeek discussed how the OMOP CDM will revolutionize the way healthcare data is being used in Europe, as it will allow the generation of evidence from observational data in a fast and reproducible manner. Peter remarked that “we are in a perfect storm,” and the upcoming IMI project EHDEN will help tremendously in the widespread adoption of the OMOP CDM throughout Europe.
Next, Nemanja Vaci (University of Oxford, UK) presented how the IMI Real world Outcomes across the Alzheimer’s Disease spectrum for better care: Multi-modal data Access Platform (ROADMAP) project tries to develop efficient uses of real world evidence for the benefit of AD patients. Adil Mardinoglu (Chalmers, Sweden) showed the audience an example of how large biological databases can help to solve very fundamental research questions to better understand disease pathologies. The last speaker of this session, Gerald Luscan (Pfizer, FR), gave a gave an overview of how the IMI European Prevention of Alzheimer’s Dementia (EPAD) project is building a platform to accelerate clinical trials and how participants are being recruited in the EPAD Register.
Perspectives on Privacy |
||||
After lunch, Valentina Strammiello (European Patient Forum, BE) gave a patient’s perspective about the re-use of healthcare data. Interestingly, while patients are genuinely concerned about data security and privacy issues, they do want bona fide researchers to be able to access their data. Patients want to be involved and to be in control of their own data. Thereafter, Nikolaus Forgo (University of Vienna, Austria) gave the audience a crash course on the upcoming general data protection regulation (GDPR), effective from May 25, 2018. Unfortunately, the GDPR will not be the hoped-for revolution to simplify the European legislation on data protection. The penalties might be higher when you are in breach with the GDPR, but the GDPR also offers reliefs and opportunities to allow the use of real world data for research purposes.
Next, there was a panel discussion on the “Past, Present, and Future of the European Health Data Ecosystem.” Panelists included Valentina Strammiello, Nikolaus Forgo, Isabelle Bos, Adil Mardinoglu, Thomas Allvin (EFPIA, BE), and Daniel Prieto-Alhambra (Oxford University, UK). Many interesting topics were discussed, such as:
- How can we do consent better?
- What’s the risk of not doing research on real world data?
- Can we keep sharing data once the GDPR is effective?
- Are we doing a good enough job of sharing the outcomes of the data with the research participants?
Projecting Forward |
||||
During the last session, we projected forward, looking at the future of the health data ecosystem. Bo Saxberg (DDO Strategic Services, US) gave a lecture about the evolving value of real world data. By augmenting the currently available real world datasets, e.g., via the inclusion of longitudinal trajectories, you tremendously increase the value proposal of the dataset for all stakeholders. Pierre Meulien (IMI, BE) then talked about the current challenges when working with healthcare data. He mentioned how IMI hopes to address these challenges in the future by focusing on the inclusion of all stakeholders, being agile, being scalable but also sustainable, and collaborating with IMI’s associated partners and other key initiatives.
For the full meeting report and the slides of the public symposium, please visit the EMIF website.
| MAY 2018 |
05 |
|||
Liberating Evidence from European Real World Data: The Odyssey of EMIF and the Legacy It Leaves |
||||
|
KEY POINTS | |||
 |
Both EMIF-AD and EMIF-MET have contributed significantly to our understanding of the natural history of Alzheimer’s disease and the metabolic consequences of obesity, as well as identifying potential biomarkers. | |||
 |
EMIF-PLAT has developed a modular architecture to support the identification, assessment, and (re)use of health data, in conjunction with an overarching governance and research process. | |||
 |
The EMIF platform will continue beyond the IMI project, led by Erasmus MC, Rotterdam, with support from Synapse Research Management Partners in Barcelona. | |||
 |
The European Health Data & Evidence Network (EHDEN), an ambitious follow-up to EMIF, is anticipated to take place 2018 – 2023.
|
|||
| CONTRIBUTORS | ||||
 |
NIGEL HUGHESEFPIA Coordinator of EMIF-PLAT, Janssen Pharmaceutica R&D  |
|||
One of the Most Cited Projects in IMI’s History |
||||
Both EMIF-AD and EMIF-MET have contributed significantly to our understanding of the natural history of their respective disease areas, as well as identifying potential biomarkers. EMIF has become one of the most cited projects in IMI’s history, and further publications will be forthcoming. Project leads for both EMIF-AD and EMIF-MET have concluded that their work, especially with regards to interaction with such large datasets, cohorts, and population data, could not have been accomplished by following a traditional route outside of EMIF. Insights into the research process with real world data have come from exploring diverse use cases, examining EMIF’s ability to respond to research questions within the two domains, and evaluating the platform architecture. Work completed as part of EMIF has also highlighted bottlenecks in the research process as well as potential socio-technical responses.
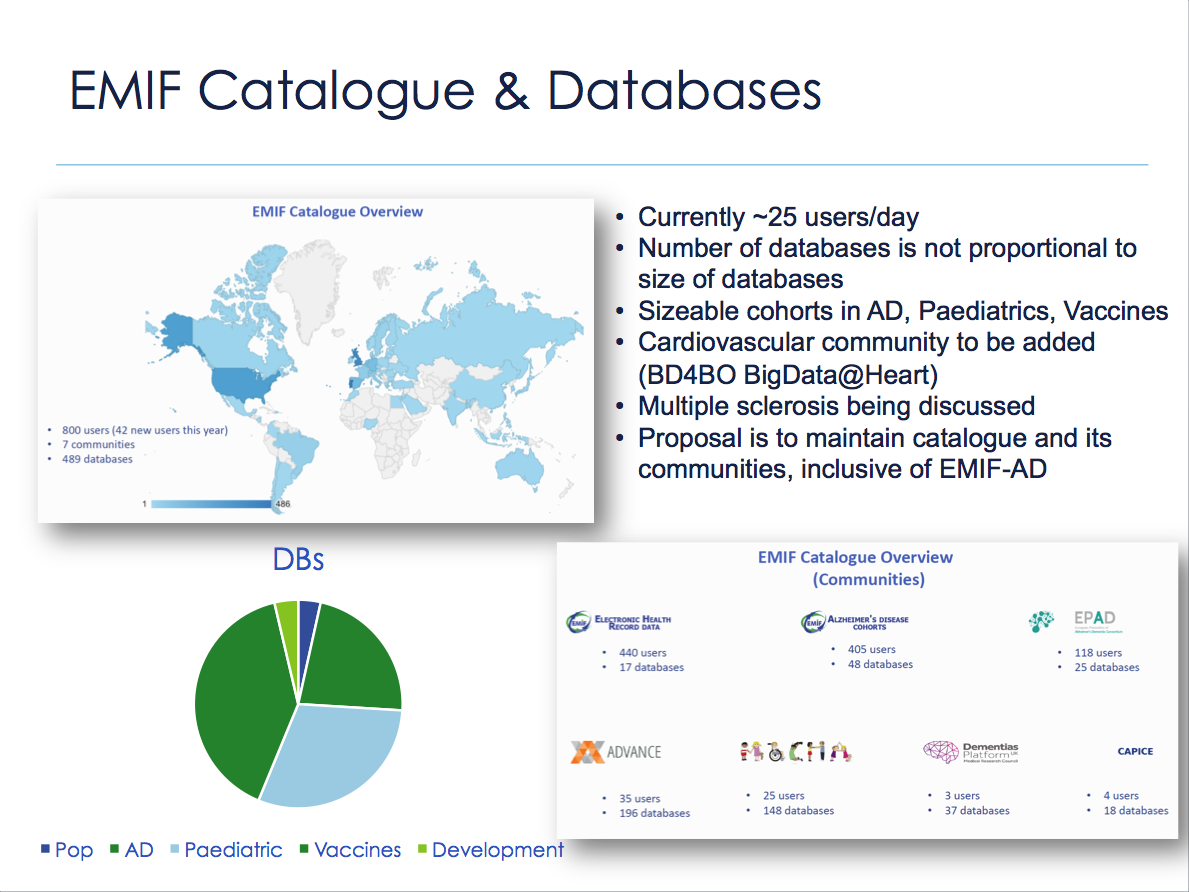
Meanwhile, EMIF-PLAT has collaborated with partner data custodians to develop a modular architecture to support the identification, assessment, and (re)use of health data, in conjunction with an overarching governance and research process. The EMIF Catalogue went live as a publicly accessible resource in January 2016, and approximately 25 researchers utilize the Catalogue each day. It houses a number of data communities, including population data sources derived from electronic health records (EMIF EHR), AD cohorts (EMIF-AD, EPAD, and DPUK), vaccine-related (ADVANCE), and pediatric (MOCHA). Additional data communities are being formed. A researcher can be granted access to a community through a Community Manager for the specific community of interest, and this accessibility will continue beyond the IMI project.
EMIF Research Process |
||||
The EMIF Platform was developed with considerable work from multiple partners in EMIF. The University of Aveiro led the architecture development, while Erasmus MC, Rotterdam, led the data harmonization. We now have an EMIF platform that can support a research process from the query hypothesis, to catalogue-driven data source evaluation, to protocol development, task management, and data extraction or analytical outputs into a private remote research environment. The local governance and provenance of the data custodian is protected within this federated network, where the processing tools are distributed to the data source instead of moving the data to a central processing hub.
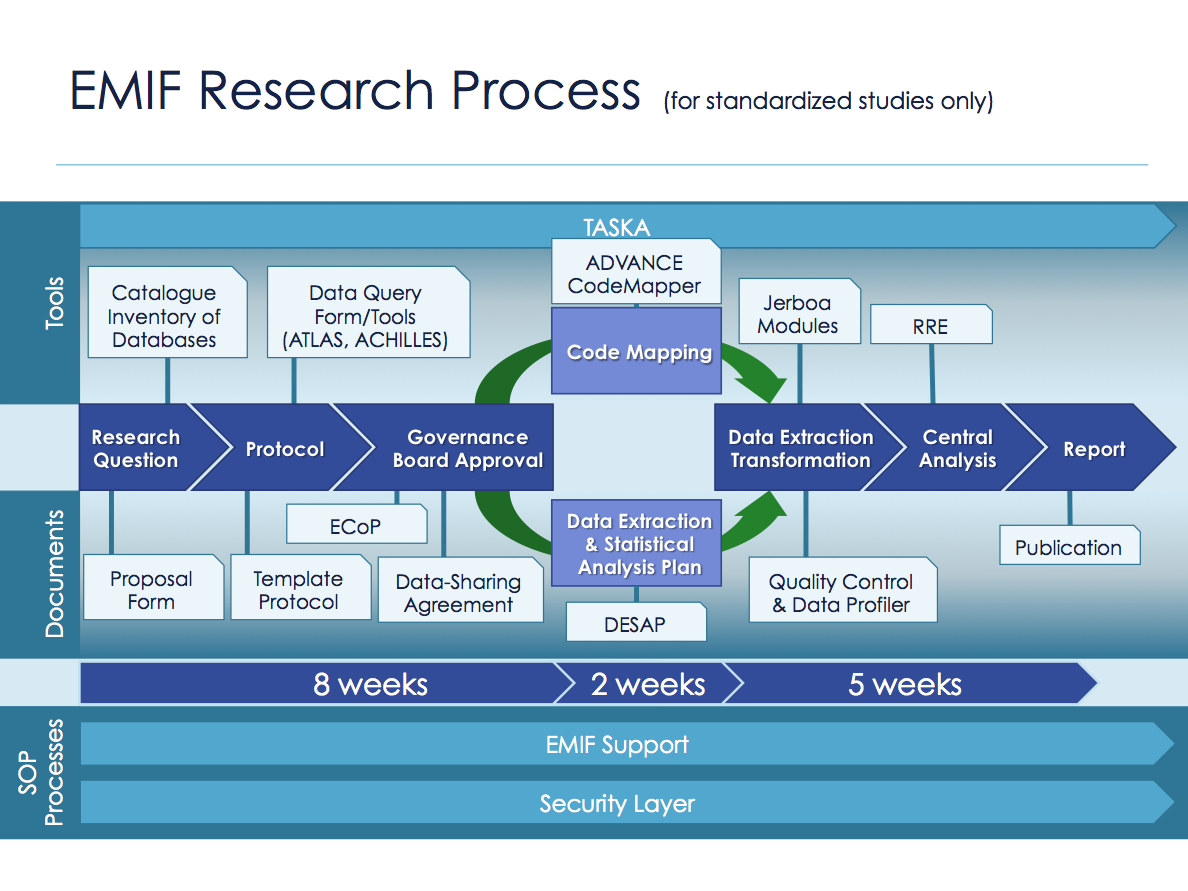
A visualization of the tools, documents, and SOP processes involved in the EMIF research process.
As part of the Observational Health Data Sciences and Informatics (OHDSI) global collaboration, EMIF has also taken a lead in promoting and utilizing the OMOP Common Data Model (OMOP CDM), as well as assisting in the launch of the EU Chapter of OHDSI. The OMOP CDM has played a critical role within EMIF, making it possible for researchers and data custodians to collaborate and harmonize data.
An Ambitious Follow-Up |
||||
The EMIF platform, based on collaboration with EHR data custodians and cohort owners in EMIF-AD, will continue beyond the IMI project. The platform will continue to support open science, open source collaboration between researchers and data custodians, and a “network of networks” of data sources. This continued collaboration will be led by Erasmus MC, Rotterdam, with support from Synapse Research Management Partners in Barcelona. The EMIF platform will also provide socio-technical input into future projects, including the European Health Data & Evidence Network (EHDEN). EHDEN is an ambitious follow-up to EMIF, currently progressing through the grant process with IMI. It’s anticipated to start before the end of 2018, again for five years. EHDEN could not have been proposed without the foundation provided by EMIF.
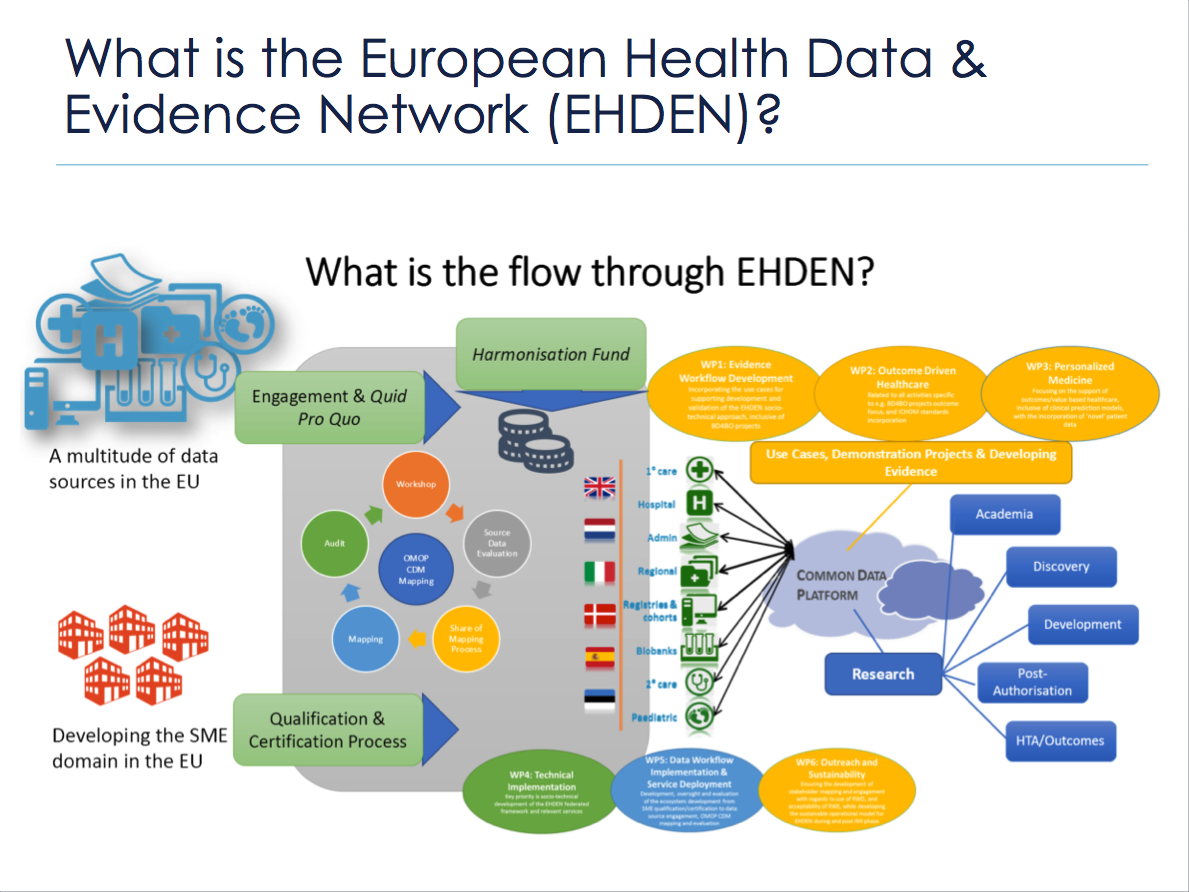
A diagram showing the flow of information through EHDEN.
To Learn More |
||||
We look forward to promoting the EMIF legacy in the remainder of the project and beyond. Anyone wishing to consider conducting research via EMIF can access the EMIF Catalogue to find the modules of the EMIF platform as well as instructional material. For further information on conducting research with EMIF, please contact Eva Molero or Montse Camprubi.





