EMIF NEWSLETTER (NOVEMBER 2017)
November 2017
Use the buttons below to jump to each article in this issue.
To Subscribe to the Newsletter, please complete the Enquiry Form.
Article 1 Improving Access to Human Health Data |
Article 2 Realizing the Value from Health Data |
Article 3 Bridging Europe |
Article 4 Celebrating Achievements and Ensuring Sustainability |
| NOVEMBER 2017 |
01 |
|||
Improving Access to Human Health Data: EMIF’s Commitment to Collaboration |
||||
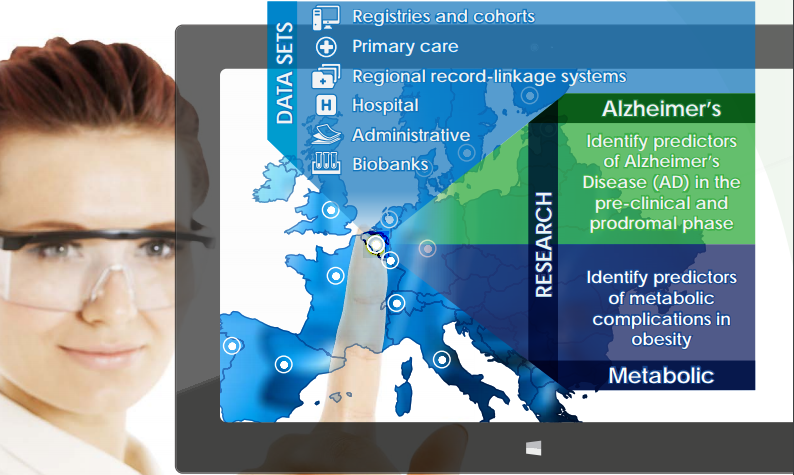
|
KEY POINTS | |||
 |
The Innovative Medicines Initiative (IMI) funded phase of the EMIF project will come to a close at the end of June 2018. |
|||
 |
In the past five years, EMIF has facilitated collaboration to make major breakthroughs in tool development, cohort development, and biomarker discovery. |
|||
 |
EMIF team members are wrapping up existing projects and also working to create a sustainable environment where collaboration will continue to flourish.
|
|||
| CONTRIBUTORS | ||||
 |
NIGEL HUGHESEFPIA Coordinator EMIF-PLAT Janssen Pharma R&D  |
|||
Collaboration Is Key |
1A | |||
Our goal was monumental, and we knew that we could only make progress by collaborating. Over the past five years (2013–2017), EMIF has brought together academic centres of excellence, subject matter experts, industry giants, and a patient organization. We have combined 57 partners across 14 European countries with €56 million worth of resources, focusing on three major projects.
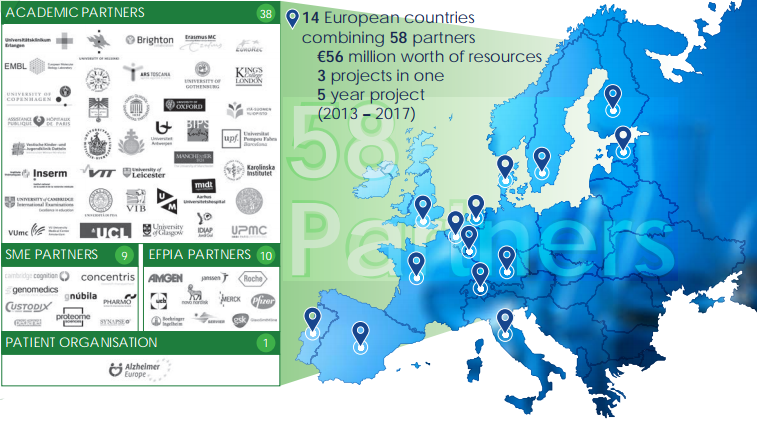
Collaboration on a grand scale has made EMIF’s achievements possible.
Major Projects and Achievements |
1B | |||
To progress toward EMIF’s goal, we developed a framework (EMIF-Platform) for evaluating, enhancing, and providing access to human health data across Europe. To help guide the development of the EMIF-Platform, we chose two pressing areas of therapeutic research: the onset of Alzheimer’s disease (EMIF-AD) and the metabolic complications of obesity (EMIF-Metabolic).
Our achievements over the past five years have been even greater than we initially hoped. At a September 2017 joint meeting hosted by the European Institute for Innovation through Health Data (i~HD) and EMIF, Professor Dipak Kalra, President of i~HD, introduced EMIF as follows: “If you wanted an example of the best in big data for medicine, EMIF would be it.” All three of our project areas have made major breakthroughs in tool development, cohort development, and biomarker discovery. We have shared these discoveries around the world, including over 117 scientific publications published, over 189 public presentations orated on EMIF, and over 75 posters presented at conferences (2013 – August 2017).
Speaking of collaboration and conferences: we are pleased to be working with Erasmus Medical Centre on the development of the Observational Health Data Sciences and Informatics (OHDSI) European Union (EU) Chapter. The first annual European OHDSI Symposium is scheduled for March 23–24, 2018, at the Erasmus Medical Centre in Rotterdam, The Netherlands. Collaborations with organizations such as OHDSI ensure that EMIF’s mission will continue even as the IMI-funded phase of the EMIF project is concluded.
Looking to the Future |
1C | |||
While this phase of our project is wrapping up, this does not mean that we can stop striving for increased access to human health data. In fact, we find that this need is growing all the time. There are countless potential applications for real world data in drug development, from discovery through development and into launch and post-launch. There will always be a need for improving life sciences research and bringing better and safer medicines to patients.
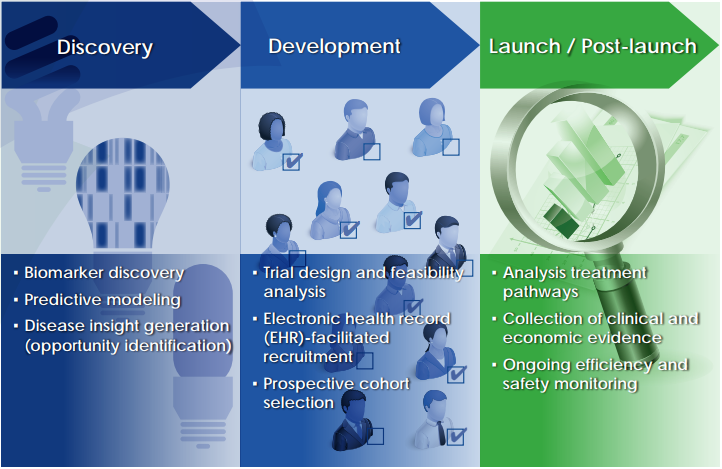
Potential applications for real world data in drug development.
As we at EMIF wrap up existing projects, we are also working to create a sustainable environment where collaboration will continue to flourish. The 10th and final EMIF-Platform consortium meeting on December 4–5, 2017, in Barcelona will focus on celebrating our achievements and ensuring sustainability. We are proud of the work that EMIF has accomplished to date, and we cannot wait to see where it leads in the future.
Contact Nigel Hughes for further information or about continuing to collaborate in the future.
| NOVEMBER 2017 |
02 |
|||
Realizing the Value from Health Data: 2017 Joint Meeting Hosted by i~HD and EMIF in Madrid |
||||

|
KEY POINTS | |||
 |
Over 200 delegates attended the September joint meeting hosted by the European Institute for Innovation through Health Data (i~HD) and the European Medical Information Framework (EMIF) in Madrid, Spain. |
|||
 |
This meeting focused on the value of reusing clinical data for improving health care and clinical research. |
|||
 |
Multiple presenters and panellists highlighted the important role of trust when reusing health data.
|
|||
| CONTRIBUTORS | ||||
 |
JELLE Project Manager at Janssen Pharmaceutica |
|||
Reusing Clinical Data |
2A | |||
The first day of the meeting was hosted by i~HD. During the kick-off session, representatives from the Spanish and Estonian health ministries elaborated on the importance of reusing clinical data for improving health care and clinical research. In addition, they emphasized the need for a dialogue with patients. They explained how essential it is to tell patients about the results achieved by allowing the reuse of their data.

Representatives from the Spanish and Estonian health ministries elaborate on the importance of reusing clinical data for improving health care and clinical research.
It’s All About Trust |
2B | |||
During the remainder of the morning, the goals and challenges of a transition toward value-based and outcomes-based health care were discussed. Mary Baker, chairperson of the session, stated, “We should stop talking about the cost of health care and start talking about the investment of health in the long run.”
The second half of the day examined the empowerment of patients. An integral aspect of patient empowerment is the development of solutions that lead to a healthy data ecosystem. This ecosystem must have quality data and also place a high priority on trust. This mutual trust in the bona fide reuse of data is of utmost importance to advance the field. As a patient representative put it: “In the end, it’s all about trust.”
EMIF Takes the Stage |
2C | |||
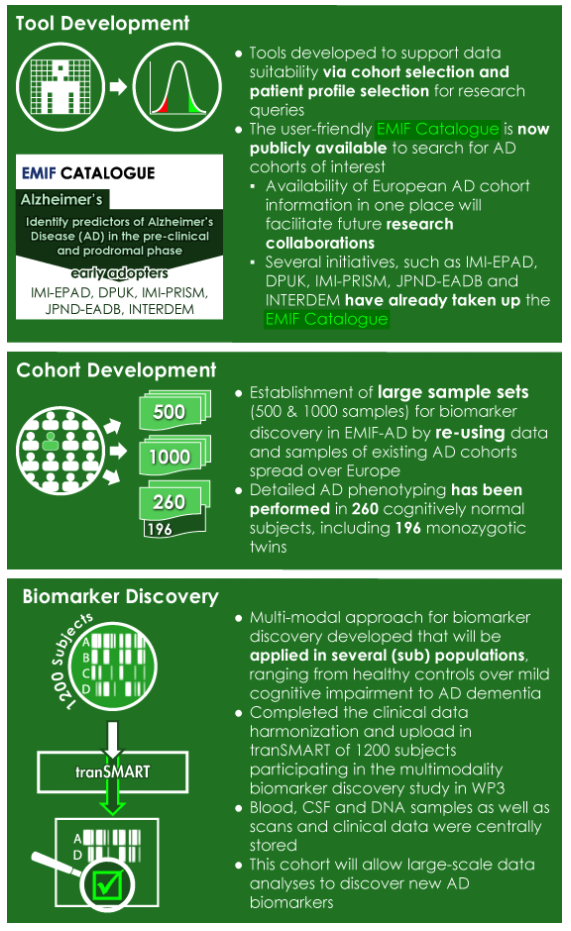
EMIF-AD Project Achievements |
For the second day of the meeting, the EMIF project took the stage. Professor Dipak Kalra, President of i~HD, introduced EMIF as follows: “If you wanted an example of the best in big data for medicine, EMIF would be it.” The day started with an overview of EMIF’s achievements in building and using the EMIF platform, and also in applying these tools to the field of Alzheimer’s and obesity. Over the past five years, EMIF has been developing a common technology and governance framework. In addition, EMIF has been creating an online platform for the identification, assessment, access, and reuse of health data across academia, industry, payers, and governments. EMIF has had two disease foci to evaluate this framework: EMIF Alzheimer’s Disease (EMIF-AD) and EMIF Metabolic (EMIF-MET). Researchers have been working to apply EMIF’s tools and technologies to identify novel biomarkers in Alzheimer’s and metabolic diseases. Both EMIF-AD and EMIF-MET have made significant achievements.
EMIF-MET Project Achievements |
For the Greater Benefit of Patients |
2D | |||
Throughout the day, presenters spoke about many different aspects of the reuse of health data, discussing each with the audience. Among the aspects discussed were technical ones, such as the harmonization of electronic health record data to the OMOP common data model (via the collaboration with Observational Health Data Sciences and Informatics, or OHDSI). Presenters also spoke about the harmonization of the AD cohort data to the Switchbox common data model which was developed within EMIF specifically for this purpose. Additionally, presenters raised many non-technical aspects with regards to governance, the expansion of data sources and collaborations, and the sustainability and legacy of EMIF elements.

Bart Vannieuwenhuyse (EMIF Coordinator; Senior Director, Janssen Pharmaceutica) speaks with an attendee during the second day of the joint meeting.
The day ended with a panel discussion. Panel members were asked what they hoped would be the legacy of EMIF. Michel van Speybroeck of Janssen Data Sciences replied, “The ultimate objective for me is that the work we put in results in improved health outcomes for everyone. I hope that we succeeded in laying the foundation for a data ecosystem in which health data can be reused in good trust and for the greater benefit of patients.”
Nigel Hughes, EMIF-Platform Co-Coordinator and Janssen Pharma R&D, added a few final thoughts to Michel’s, saying, “80% of bioinformatics is about humans, not the data itself.” Professor Sir Simon Lovestone, EMIF Co-Coordinator and professor of translational neuroscience, Oxford, agreed: “If we can get patients and all partners involved to trust each other, and we couple this to our endless ambition, we can really make a difference in patients’ lives.”
| NOVEMBER 2017 |
03 |
|||
Bridging Europe: First Annual European OHDSI Symposium Scheduled for March 2018 |
||||

|
KEY POINTS | |||
 |
The European Medical Information Framework (EMIF) has adopted the Observational Medical Outcome Partnership Common Data Model (OMOP-CDM), developed by the Observational Health Data Sciences and Informatics (OHDSI) Initiative, to standardize European data sources to a common structure and language. |
|||
 |
EMIF is working with Erasmus Medical Centre to develop the European OHDSI Chapter. |
|||
 |
The first annual European OHDSI Symposium will be on March 23–24, 2018, at Erasmus Medical Centre in Rotterdam, The Netherlands.
|
|||
| CONTRIBUTORS | ||||
 |
PETER Assistant Professor, Department of Medical Informatics at Erasmus University Medical Centre |
|||
Data Standardization to the Rescue |
3A | |||
How did EMIF overcome this challenge? By embracing the Observational Health Data Sciences and Informatics (OHDSI, pronounced “Odyssey”) model of translating the data into a common structure and language. You might recognize this concept—it’s known as “data standardization.” We at EMIF have been mapping European data sources to the common data model developed by OHDSI. We are also extending OHDSI tools to enable the large-scale analytics that are key to EMIF’s core objectives.
The Origins of OHDSI |
3B | |||
OHDSI grew out of the Observational Medical Outcomes Partnership (OMOP), a public-private partnership originally established in the United States. The OMOP project developed the initial version of the OMOP Common Data Model (OMOP-CDM), which can accommodate health data from diverse sources in a consistent and standardized way.
OHDSI’s mission is to improve health, by empowering a community to collaboratively generate the evidence that promotes better health decisions and better care. A key objective is to stimulate transparent and reproducible research for which the OMOP-CDM is a prerequisite.
Today, OHDSI is an international collaborative comprised of academics, industry scientists, healthcare providers, and regulators who have come together to create and apply open-source data analytic solutions to a large network of health databases. It’s a non-funded entity, supported by the generosity and passion of individual members as well as in-kind contributions from many organizations. OHDSI is powered by a spirit of global collaboration.
A New Chapter for OHDSI |
3C | |||
We at EMIF are pleased to be working with Erasmus Medical Centre on the development of the European OHDSI Chapter. The first annual European OHDSI Symposium is scheduled for March 23–24, 2018, at Erasmus Medical Centre in Rotterdam, The Netherlands.

The Erasmus Medical Centre in Rotterdam will host the first annual European OHDSI Symposium (source: EGM Architecten).
This symposium will be called “Bridging Europe.” It aims to establish a European community that actively contributes to the global OHDSI mission. At the symposium, participants will share results and ideas about the use of the OMOP-CDM, tool development, and future research studies. The meeting will feature a collaborator showcase involving a poster session to highlight OHDSI’s research achievements and interactive demonstrations of OHDSI’s open-source software tools.
“Standardizing our European health data will enable distributed research studies at an unprecedented scale,” says Dr Peter Rijnbeek, Assistant Professor in the Department of Medical Informatics at Erasmus University Medical Centre. “The annual OHDSI symposium is the perfect instrument to stimulate and support this transition. Join this exciting journey!”
Register Now |
3D | |||
Have you been an active participant in EMIF’s work utilizing the OMOP-CDM? Or are you new to OHDSI and interested in learning more about this exciting initiative? Whatever your level of experience, you are welcome. Registration is limited to 250 participants, so please register as soon as possible to reserve your spot.
| NOVEMBER 2017 |
04 |
|||
Celebrating Achievements and Ensuring Sustainability: The 10th and Final EMIF-Platform Consortium Meeting |
||||
|
KEY POINTS | |||
 |
The European Medical Information Framework (EMIF) Platform community will gather for a final consortium meeting on December 4–5, 2017. |
|||
 |
The meeting will focus on how learnings from Use Cases might be taken into consideration for the final Platform. |
|||
 |
Another topic at the meeting will be sustainability, including publication strategy, business planning, and legacy. |
|||
 |
EMIF needs to work inclusively to progress the sustainable elements that will continue beyond the Innovative Medicines Initiative (IMI)–funded phase of the project.
|
|||
| CONTRIBUTORS | ||||
 |
NIGEL HUGHESEFPIA Coordinator EMIF-PLAT Janssen Pharma R&D  |
|||
Looking Forward |
4A | |||
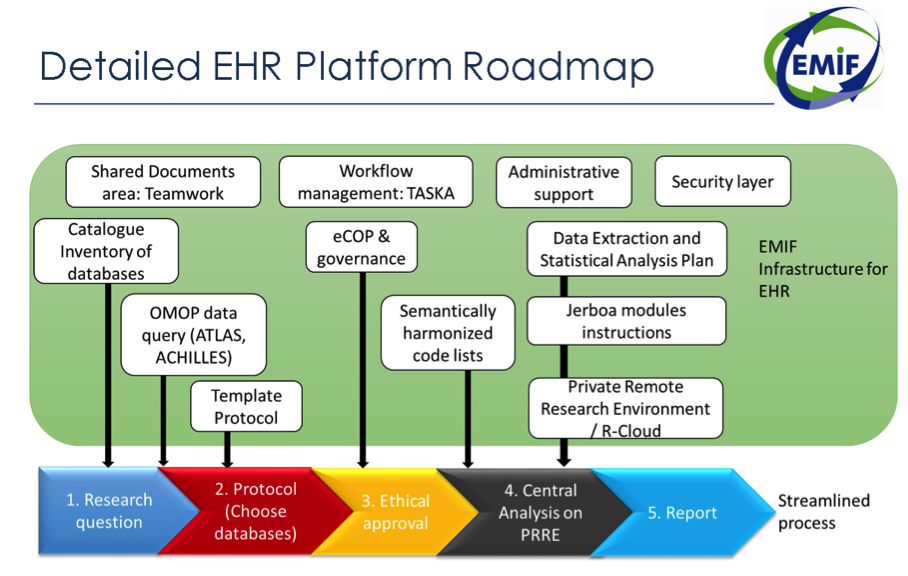
At the 9th consortium meeting in Barcelona in May 2017, many important milestones were discussed, including the technology platform, data harmonization, sustainability, research use cases, expedited research efficiency, and publication strategy. The EMIF-PLAT community will gather for a final consortium meeting on December 4–5, 2017. At this 10th and final consortium meeting, members will evaluate progress to date as well as discuss final activities up until the end of the project in Jun 2018.
Our 10th meeting will likely reflect on one of our most well-known achievements: the EMIF Catalogue going public in January of 2017. We now have over 400 users registered in the EHR community of the Catalogue. Since January of 2017, we have continued to work on data harmonization with the electronic health record data custodians via the OMOP Common Data Model. This has involved further work evaluating data fidelity.
The EMIF Catalogue homepage.
EMIF’s collaboration with OHDSI has made much of our data harmonization work possible. We are pleased to be working with Erasmus Medical Centre on the development of the OHDSI European Union (EU) Chapter. The first annual European OHDSI Symposium is scheduled for March 23–24, 2018, at Erasmus Medical Centre in Rotterdam, The Netherlands.
Onboarding New Cohorts |
4B | |||
At our 10th meeting, we will also evaluate our work with additional EMIF Alzheimer’s disease project (EMIF-AD) cohorts. Using the Switchbox common data model, we have onboarded these cohorts into the EMIF-Platform. While onboarding new cohorts, we make sure that they populate the participant and the variable selection tools. These tools allow researchers to identify subsamples of individuals and subsets of variables that fit the needs of their research questions. All of this work supports the establishment of the EMIF-AD platform’s sustainable infrastructure, which is crucial for AD research in Europe.
Expedited Research Efficiency |
4C | |||
Our consortium meeting will explore how to manage research more efficiently. We are currently considering the potential use of a commercial-like study to evaluate the whole EMIF-Platform—from Catalogue to private remote research environment—prior to the end of the EMIF project. In order to expedite research efficiency, we have also identified bottlenecks in the research process that need to be addressed, as well as the technology supporting research via the EMIF-Platform.
Prioritizing Publications |
4D | |||
Since the meeting in May, the EMIF-PLAT community has been working on further publications from the project. We look forward to the fact that the 10th consortium meeting will include discussions of members’ submissions to relevant journals.
An excerpt from the extensive publications list on the EMIF website.
Working Inclusively |
4E | |||
There is considerable work to do prior to the official end of the EMIF project at the five-year mark. We need to work inclusively to progress the sustainable elements that will continue beyond the IMI-funded phase. We are excited about the upcoming consortium meeting where we will celebrate work achieved, shepherd the project to a successful conclusion, and solidify plans to sustain the EMIF-Platform in the future.



